Day: November 21, 2024
-
Chapter 15 Solved Exercise Organic Chemistry
Explore comprehensive chemistry resources for Federal Board students! This section covers essential topics like functional groups, alkyl groups, isomerism, reaction mechanisms, homologous series, and bond fissions. With detailed explanations, structural...
-
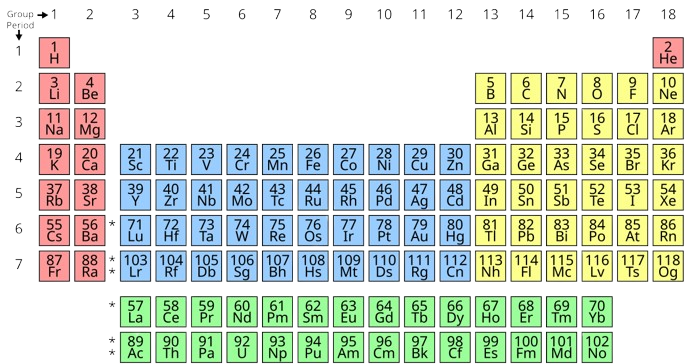
Chapter 10 Periodic table Solved Exercise
Master the fundamentals of periodic table trends and reactions with this detailed guide. Explore key concepts, including ionization energy, electron affinity, and metallic vs. non-metallic character. Learn how sodium and...